In 1964 a Supreme Court Justice was attempting to decide whether the film “The Lovers”, directed by Louis Malle, was pornographic. He struggled in his attempts to define pornography. In the end he said that he couldn’t really define what it was, but he “knew it when he saw it”. Nowadays, we might say the same thing about what we mean by the word “drug”. How about aspirin or morphine? Are they drugs ? No question about it. What about sugar, also a white powder that we consume? Probably not. How about ziconotide, a 25 amino acid peptide that is injected into the intrathecal space of the spinal cord for the treatment of chronic pain? Yes, I think so. What about nusineresen, an antisense oligonucleotide that is also injected into the intrathecal space for the treatment of Spinal Muscular Atrophy (SMA)? A bit less clear, but I think I might go out on a limb and say that it is a kind of drug. What about Chimeric Antigen Receptor engineered T-cells (CAR-T cells) for treating acute lymphoblastic leukemia (ALL)? A fantastically interesting therapeutic advance but maybe not a “drug” in the traditional sense. Originally a drug was usually considered to be a small organic molecule that might be taken in crude form if it was a natural product (e.g. opium) or in its purified form (e.g. morphine). It might be taken as a pill, injected or drunk (e.g. laudanum). Nowadays, there are more and more types of molecules that can act as therapeutic agents in medicine. So, perhaps our definition of a drug needs to be revised?
But just how far do we need to expand the definition? The first drugs were all natural products. From time immemorial humans have availed themselves of substances that they found in plants and animals. Drugs like morphine and cannabis have a very long history of use by humans for medical and religious purposes as described in the ancient works of Galen, Dioscorides and others. However, the most significant advances in pharmacology really took place in the 19th century with the arrival of the science of organic chemistry, which provided mankind with new ways of purifying and modifying natural products. Now, morphine could be purified from opium and, furthermore, it could be subjected to chemical reactions like acetylation to produce an entirely new molecule, diacetyl morphine (heroin). And so the discovery of new drugs became much less haphazard. Furthermore, our ability to invent and manufacture novel drugs gave rise to a new type of industry -the pharmaceutical industry.
The story of the pharmaceutical industry began with the efforts of a young English student named William Perkin. Perkin had enrolled in England’s first professional school of chemistry, the Royal College of Chemistry, in 1853 when he was only 15 years old. Three years later, his professor suggested that he might attempt to produce quinine from the simple organic chemical aniline . Perkin started to carry out his “homework” in his home laboratory. Quinine was a drug that was of great interest at the time owing to the fact that it was the only thing that could treat malaria which was endemic in many parts of the world. However, quinine was produced from the bark of the cinchona tree which only grew in Peru and was therefore difficult to obtain. Synthetic quinine was the obvious solution. One reaction Perkin carried out, treating aniline with potassium dichromate, left him with a black sludge at the bottom of his glass flask. However, once he shook this residue up in alcohol it turned a beautiful purple colour. Struck by this, Perkin wondered if this purple liquid would dye cloth. It did. He had created the first synthetic dye which he named “mauve”. Indeed, Perkin had made a paradigm shifting discovery because, prior to this time, all dyestuffs had to be isolated from natural sources such as plants, and, like the production of quinine, this was a very labor intensive process. A new industry, the “chemical industry” resulted from Perkin’s discovery. New chemical companies grew up, particularly along the river Rhine in Germany, and created a multitude of new synthetic aniline based dyes and other products. Some of these substances proved to have interesting beneficial effects when administered to animals or humans and so some of the chemical companies became pharmaceutical companies, producing new drugs for treating many diseases including malaria.
In fact, the connection between synthetic dyes and drugs was something that would continue to produce important ideas for the future of pharmacology. It turned out that new dyes weren’t just useful for producing coloured materials for clothing. Generally speaking, it was observed that certain dyes would stain some biological materials but not others, allowing them to be readily identified, so that details of their structure could be better discerned under the microscope. For his PhD thesis in 1878 a young German student named Paul Ehrlich began to use dyes for identifying different types of cells in the blood including the first identification of mast cells. He observed that different dyes could specifically stain different cells ranging from mammalian cells to protozoa and bacteria. From results such as these Paul Ehrlich developed what would become a very influential idea. If dyes could selectively interact with cells through molecules attached to their surfaces might they be also able to seek them out and destroy them like “magic bullets”? It had been observed that the dye methylene blue could selectively stain malaria parasites. So, as a test of his idea,Ehrlich decided to try using the dye to treat malaria patients. In 1891 he administered methylene blue to two patients, a domestic servant and a sailor. Their condition seemed to improve greatly. There were certainly problems, such as the fact that the whites of their eyes turned blue. Nevertheless, buoyed by his success Ehrlich continued to perform antimalarial trials with methylene blue in collaboration with the Bayer drug company. Subsequently, several other dyes, were used to treat diseases like malaria and African sleeping sickness with some success. Sleeping sickness is caused by an organism called a trypanosome, reflected in the names of the dyes trypan red and trypan blue which were designed to treat the disease.
In fact, a version of Ehrlich’s magic bullet theory had been in play since the time of the great alchemist Paracelsus. In 1530 he suggested that diseases might be specifically targeted by metals. For example, syphilis, a terrible problem in Europe at the time, might be treated with mercury. This is the basis of the famous saying –“One night with Venus: a lifetime with Mercury”. By the 19th century arsenic was also being widely used for treating syphilis. Although some benefit might be obtained by these treatments, things like mercury and arsenic were also horribly toxic, often being just as bad as the disease they were designed to treat.
The birth of organic chemistry in the 19th century enabled the synthesis of organic derivatives of arsenic and mercury to be made in an attempt to temper their toxic side effects.

An organic arsenic derivative called atoxyl had been produced as early as 1863 and had found some use in the treatment of trypanosome induced sleeping sickness. Ehrlich originally thought that syphilis was also caused by a trypanosome and so decided to try modifying atoxyl. Erlich’s laboratory synthesized several hundred derivatives of arsenic with a view to producing new drugs for targeting syphilis in particular. In 1905, Fritz Richard Schaudinn, a German zoologist, and Erich Hoffmann, a dermatologist, discovered Spirochaeta pallida (the bacteria was spiral shaped and white under dark ground illumination, now called Treponema pallidum) to be the organism that caused syphilis. Ehrlich, therefore, developed a model for testing new drugs involving the infection of rabbits with T.pallidum. In 1909, following a systematic testing of the new molecules provided by his colleagues, Ehrlich eventually hit upon “compound number 606”, which he subsequently named arsphenamine and which effectively cured syphilis in rabbits with a single dose. The drug was effective against various bacterial strains of Spirochaetes, findings that led to clinical trials and eventual marketing of the drug for the treatment of syphilis. There was no FDA or its German equivalent in those days and the new drug, now named Salvarsan, rapidly made its way into the clinic and was being used to treat humans with great success in 1910. Salvarsan was certainly an enormous advance on the use of mercury or arsenic. Manufactured by the German chemical company Hoechst, Salvarsan quickly became the most widely prescribed drug in the world. It was the world’s first blockbuster drug and remained the most effective drug for treating syphilis until the 1940s. Not only was Salvarsan an important drug but it was also provided clear support for Ehrlich’s entire approach to pharmacology, testing of a series of novel molecules produced through gradual modifications of lead compounds using organic chemistry and testing them in disease relevant assays, in search of the ultimate “magic bullet”. Really, the whole of modern pharmacology dates from Ehrlich’s work with Salvarsan which provided a paradigm for drug discovery that has been used ever since. However, although Salvarsan, and its subsequent derivatives, were successful in treating many cases of syphilis, they were anything but ideal from a modern perspective.
Ehrlich’s approach and the dye/drug connection would bear further fruit. In November 1936 Franklin D. Roosevelt (FDR) Jr., the son of the US president, had been taken to hospital. What had started as a sinus infection began to go rapidly downhill. FDR Jr. was suffering from a Streptococcal infection that formed an abscess in his cheek and spread to his throat. His temperature rose to very high levels and he began to cough up blood. If the infection entered his blood, producing a general systemic infection known as sepsis, it was quite possible he would die. The situation was critical. Dr. George Loring Tobey Jr, the otolaryngologist involved in the case had an idea. He would try a new experimental drug which he had previously tested on some of his most serious patients. The drug was called Prontosil Rubrum or Prontosil Red. True to its name, it was a bright red dye that had been developed by a scientist at the IG Farben company in Germany as part of their program for developing drugs that could fight microbial infections.
Dr. Tobey gave FDR Jr several injections of the bright scarlet dyestuff. The results seemed quite miraculous. After 3 weeks in the hospital, sinking deeper and deeper into crisis, his condition completely turned around. His temperature started to come down, his strep throat improved and the abscess in his cheek started to shrink. He clearly began to feel better. This was in spite of the fact that his skin now appeared a bright tomato color-a small price to pay under the circumstances. In a few days, he was released from hospital and his skin gradually regained original healthy colour. The story of his cure created a sensation in the media.
Prontosil Red had been developed by a German pharmacologist named Gerhard Domagk who was working in a program at the Bayer drug company that was descended from Ehrlich’s original work. By the 1930s the major German chemical and pharmaceutical companies, including Bayer, Agfa, BASF, Hoechst and Casella, had consolidated themselves into a giant cartel named IG Farben (Interessen Gemeinshaft Farbenindustrie: The combined interests of the dye making companies), thereby becoming one of the 5 largest companies in the world. By tweaking the chemistry of dyestuffs, Bayer chemists, now working for IG Farben, had already synthesized mepacrine in 1931, a drug that became one of the first widely used alternatives to quinine in the treatment of malaria. Domagk and his colleagues were charged with finding similar dye related substances that were active against streptococcal infections and he had devised an assay system for testing newly synthesized molecules using infected mice. Having not got very far with the program by modifying traditional aniline based dyes, the chemists at IG Farben switched track and began making a series of substances based on azo-dyes. In late 1932 they started to produce azo-dyes that contained the chemical sulfonamide nucleus and passed these on to Domagk for testing. These were much more promising. One in particular produced dramatic effects in mice infected with deadly Streptococcus. Fourteen mice given the drug all survived whereas another group who didn’t receive it all died. Indeed, this was the drug that would eventually become known as Prontosil Red.
The scientists at IG Farben soon confirmed that Prontosil Red was the real deal. Indeed, one incident confirmed this in the most dramatic fashion imaginable. In 1935, Gerhard Domagk’s young daughter Hildegard stabbed herself with a dirty needle, which led to a Streptococcal infection. As we have seen in the case of FDN Jr., this was something to be taken very seriously indeed. Hildegard’s temperature rose to 104 degrees as the infection spread to her blood and her outcome looked bleak. Domagk arranged for his daughter to be treated with Prontosil Red and within a week she was completely cured. Further studies that he carried out convinced Domagk that Prontosil Red was active against other infections as well, including those that caused bacterial meningitis and gonorrhea. It appeared that the IG Farben scientists had made a breakthrough of historic importance that would not only help humanity treat a series of previously untreatable diseases but would also bring the company a windfall of profits. The first of these possibilities became reality but, alas for IG Farben, not the second.
In fact, right from the start there had been something curious about the way in which Prontosil Red worked. It was extremely effective in animals, including, as we have seen humans, but it was completely inactive when added to bacteria in a culture dish or test tube. A group of scientists in France carefully read the IG Farben patent literature and came up with a way to produce their own version of Prontosil Red which they named Rubiazol, and used this as a starting point for making their own derivatives for testing. Many of these drugs were effective. As a control experiment, they then tested the sulfonamide moiety which was common chemical component of all of the effective dye molecules. This was a substance called sulfanilamide. It turned that sulfanilamide, when given just by itself, was at least as effective as substances like Prontosil/Rubiazol. This made everything clear. Drugs like Prontosil Red were actually acting as ‘Pro-drugs”. They had no real antibacterial effect but once inside the body an enzyme would cleave them in half, releasing sulfanilamide, which now produced the beneficial effects observed. However, sulfanilamide had been synthesized and patented in 1906, meaning that IG Farben ,who had been responsible for revealing its profound antibacterial effects, were left with nothing to show for it. Nevertheless, sulfanilamide was the first of a group of similar substances with genuine antibacterial effects. Sulfa drugs were the first real generally useful “antibiotics” which could be used for fighting a number of important previously untreatable infections. They became widely used and proved extremely effective for treating the wounds of soldiers in the Second World War. Of course, the subsequent story of the discovery and development of antibiotics obtained from natural sources including such drugs as penicillin and streptomycin is one of the most important in the history of medicine leading to many Nobel Prizes including awards to Alexander Fleming, Selman Waksman and others.
The problem with antibiotics these days is that, after years of misuse, we are now faced with the fact that many dangerous pathogens have become resistant to the effects of these drugs. The situation is so serious that we are faced with a scenario in which mankind will once again be at the mercy of the same infectious diseases as it was prior to the advent of sulfa drugs and other antibiotics. New approaches and novel types of “drugs” are required. There are some interesting possibilities. Consider the case of Dr. Tom Patterson, a professor of psychiatry at the University of California in San Diego, who was on vacation with his wife in Egypt in 2015. At some point during his visit he became violently ill with symptoms including abdominal pain, fever, nausea, vomiting and a racing heartbeat. Local doctors figured out that he had an infection of the pancreas but couldn’t seem to treat him with the drugs they had on hand, and so he was rapidly transferred to an American hospital in Frankfurt, Germany. Here the doctors identified the infectious pathogen as multidrug resistant Acinetobacter baumannii, which has emerged as a highly antibiotic resistant organism which is extremely dangerous. Eventually, it was found that if he was treated with a combination of the antibiotics meropenem, tigecycline and colistin, the latter being rather nephrotoxic and so considered a treatment of “last resort”, his condition did stabilize somewhat and he was transferred back to the ICU at the UC San Diego hospital. Here he continued to slowly get better. Unfortunately, an accident occurred which caused the bacterium to enter his blood stream and he immediately began to suffer the symptoms of septic shock. The antibiotics being used to treat him were no longer effective under these circumstances and he became comatose. It was clear he was going to die. However, his wife, also a doctor, was determined to find something that might help.
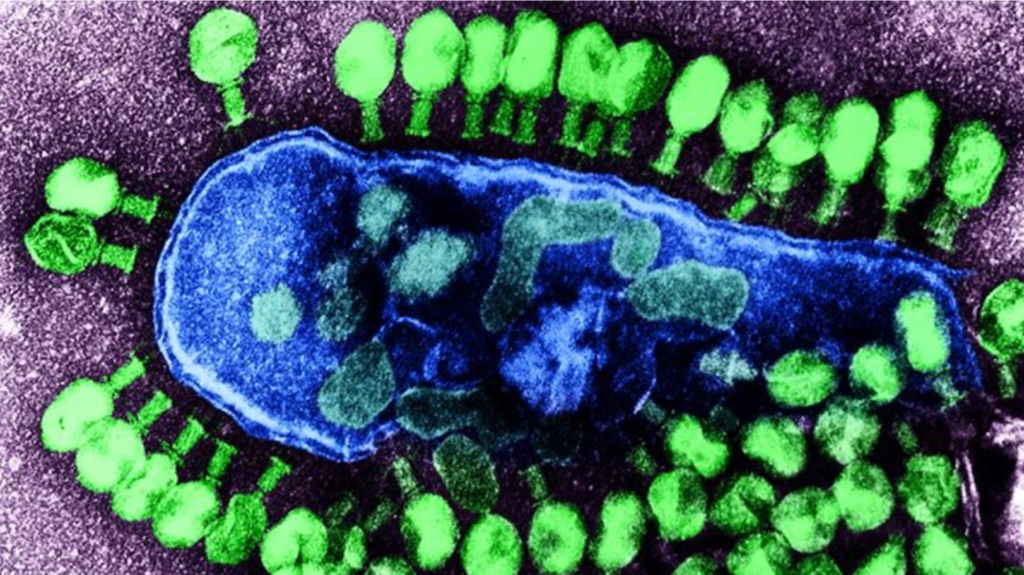
To understand what eventually happened one has to go back to a time before antibiotics had been introduced for the routine treatment of infectious diseases. In 1915, Frederick Twort, superintendent of the Brown Institution of London, discovered something that could infect and kill bacteria. The substance consisted of very small particles and could easily pass through most filters. Similar observations in France by Félix d’Hérelle, working at the Pasteur Institute in Paris, led to the realization that the material was actually a virus which was named a bacteriophage from “bacteria eating virus”- organisms that are commonly referred to as “phages”. Phages are common to say the least. There are more than 1031 bacteriophages on the planet-that is ten million trillion trillion or “rather a lot”. In fact, there are more phages than every other organism on Earth, both large and small, combined. Because of the fact that phages naturally kill bacteria there was considerable research in the 1920s and 1930s attempting to use phages as a method for treating bacterial pathogens and indeed, these attempts met with some success by the standards of the time. However, once antibiotics were discovered research on “phage therapy” virtually stopped in the USA and Western Europe. Why bother when you could just use a wonder drug instead? Interestingly, the research did continue to some extent in the Soviet Union, particularly in the Soviet republic of Georgia. Nowadays, however, with the rise of antibiotic resistance there has been something of a resurgence in the idea of phage therapy. Because there are a lot of different types of bacteria and a lot of different phages it is necessary to find the correct phage for a particular bacterium or, another possibility, to give a cocktail of phages in the hope that one of them will hit the target.
This is precisely what happened in the case of Tom Patterson. With her husband lying in a coma waiting to die from infection by an organism that was completely resistant to all known antibiotics, Tom’s wife Steffanie had to think fast. Steffanie, who had a PhD and was an expert on Global Health, remembered that she had heard of people who had antibiotic resistant infections traveling to Soviet Georgia and being cured by phage therapy where it was still being used. She quickly contacted several institutions that had worked on phages that were specific for the organism that had infected Tom Patterson. Once these had all been collected in San Diego and purified they were intravenously injected into the patient. Just as with Franklin D Roosevelt Jr. all those years previously being tested with a new therapy, the effects on Tom Patterson were dramatic. Within 3 days he was out of his coma and much recovered. In fact, the path to his recovery was not altogether a simple one. The bacterium began to mutate and evolve within Tom as it became resistant to the effects of one phage cocktail, necessitating the use of another and so on. But eventually the doctors were victorious, the bacterium was vanquished and Tom emerged emaciated but cured. This result which has been described as “a game changer” in the field has led to the founding of The Center for Innovative Phage Applications and Therapeutics (IPATH) at the University of California, San Diego which is specifically dedicated to phage therapy.
So is a phage a drug? It certainly fits Ehrlich’s concept of a magic bullet. Indeed, Ehrlich also considered things like antibodies, which are increasingly used today as therapeutics, to be magic bullets. Maybe it’s just not worth arguing about the definition of a drug and use common sense. We know it when we see it.
