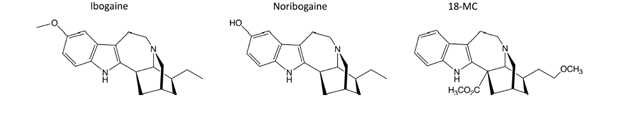
Recent media coverage of the Opioid Crisis has highlighted our historically two-edged relationship with opioids. Drugs like morphine are clearly the most effective agents for dealing with many types of pain. They are essential for most surgical procedures and, when used appropriately, they are our most effective drugs for treating many acute injuries or other causes of pain. On the other hand, opioids are dangerous to use, a high dose will kill you by stopping your breathing, and they are extremely addictive. The results of the unrestricted availability of opioids became clear during the first Opioid Crisis which occurred in China in the 19th century due to the enormous opium burden foisted on the Chinese by western powers, particularly the British. The result was a social catastrophe. The current situation in the USA is another version of the same scenario, although in this case the opioid suppliers were drug companies and doctors rather than aggressive merchants. Because we have always been aware of these potential problems, drug makers have tried to produce novel, problem-free opioid drugs since the 19th century. Indeed, heroin, first marketed in 1898 by the Bayer drug company, was originally sold as a non-addictive alternative to morphine—clearly rather an unfortunate error. Since that time, there have been thousands of attempts to produce non-addictive opioids based on all kinds of strategies ranging from lower agonist efficacy to biased receptor signaling. These attempts inevitably produce high-profile scientific publications in journals like Nature or Science but, unfortunately, have yet to yield any benefit in the human population. Smaller piecemeal approaches such as the use of methadone, suboxone or opioid antagonists have made some differences but a knockout punch is still to be delivered. Strangely, one of the most promising treatments for opioid abuse disorders may have been hiding in plain sight. The name of the drug is ibogaine. As I will discuss, ibogaine itself is unlikely to ever come to market as a treatment for substance abuse. Recently, however, a new company called MindMed announced that it was starting phase II trials on a substance called 18-MC which is closely related to ibogaine and may share a similar mechanism of action. But what is 18-MC and why might it be important?
In many respects it’s an old story because ibogaine has been around for a long time, flitting in and out of our collective consciousness. During the 1960s and 1970s, several classes of psychotropic drugs became illegal following the passage of the Controlled Substances Act and similar laws. This included psychedelic drugs such as LSD, psilocybin and mescaline. Laws that made cannabis illegal had been passed prior to this time, stretching back to the 1930s. Nevertheless, there was a great deal of scientific evidence that both cannabis and the psychedelics might have important medical benefits. Whatever the truth of the matter, after 1970 research into the potential uses of these types of drugs generally ceased. During the past decade however, there have been signs that interest in these substances is reviving and that changes in the laws are making it easier to investigate them once again. Although cannabis is still illegal at the federal level, many states have gone their own way and have legalized its medical or recreational use once again. Recently there have been signs that psychedelic drugs such as psilocybin may be undergoing a similar renaissance. These events have been widely covered in the press and most people will be aware of at least some of the things that are going on.
The stories of all of these drugs have followed a somewhat similar path. Originally discovered by ancient peoples and used for religious purposes, they were “rediscovered” by western societies and were taken up by both the pharmaceutical industry and by many people for recreational use. All of these drugs are related to natural products derived from plants found in Europe, Asia and South and Central America. But what about Africa? The African continent certainly has its fair share of interesting plants and natural products which, as in other parts of the world, originally saw the light of day in the context of religious practice. For example, in western parts of Africa around Gabon and Cameroon there are large numbers of people, particularly among the Fang population, who practice a religion called Bwiti—a syncretic combination of indigenous animist beliefs and Catholicism. An important feature of Bwiti religious practice is the use of Tabernanthe iboga, a hallucinogenic shrub. The bark of the roots of the plant produces powerful psychotropic effects and is used in religious ceremonies. The drug is described as producing visions and a state of lucid dreaming as well as long-lasting introspection. On the other hand, unlike other psychotropic drugs such as LSD, the experience of taking ibogaine is not reported as being a particularly pleasant one—more like hard work, which has limited its use as a recreational drug. According to the Fang themselves, the discovery of the psychoactive properties of the plant goes back to their encounters with the Pygmies, who were very knowledgeable about the properties of plants in the African rainforest. This knowledge was passed on to the Apindji and the Mitsogo peoples as they migrated into west Africa and originated the Bwiti faith. The Bwiti practice was then passed on to the Fang people in the 1890s, and they have established a complex mythology around the use of T. iboga. Consider this quote: “You have heard what the Catholics tell us regarding a fruit that our first parents ate. What kind of fruit did our first parents think they ate, Adom-Obola and Eve-Biome? What type of tree was it? They are lying because they do not want to tell us the truth. For this reason, God left the iboga so that men would see their bodies as God had made them, as He himself has hidden inside them. Therefore brothers, take the iboga plant that God gave to Adam and Eve.”
When Bwiti shamans consume T. iboga, they believe that they gain the ability to heal the sick, communicate with the dead, and experience visions of the future. Perhaps most significantly, the drug is crucial to the initiation rites and coming-of-age rituals of the Bwiti religion.
T. iboga was first introduced to the West in 1864, when samples of the plant were brought back to France from Gabon by French anthropologists who visited the region as part of expanding French colonial influence. The first publication in the literature as to its ritual use appeared in 1885. Although the plant contains a large number of chemically related pharmacologically active substances, the major component, known as ibogaine, was first crystallized from extracts of the shrub’s root bark in 1901. The introspection that results from taking the drug meant that it was subsequently developed for use in psychiatry as an aid to psychotherapy. From 1939 to 1970, ibogaine was marketed in France under the trade name “Lambarene,” a “neuromuscular stimulant” in the form of 8-mg tablets, a fairly low dose, for conditions including fatigue and depression. However, as discussed above, by 1970 the effects of the counter cultural movement had prompted all western governments to make any kind of drug with hallucinogenic properties illegal. This included ibogaine which, in the USA, became a Schedule 1 drug. This meant that along with the likes of heroin, cannabis and LSD, it was supposed to be extremely dangerous and to have no medical utility whatsoever. Nevertheless, this was not to be the drug’s ultimate fate.
The history of ibogaine in the USA makes an interesting story. It is well known that in the 1950s and 60s the CIA had a large “secret” program variously called BLUEBIRD, ARTICHOKE or MK-ULTRA tasked with discovering mind-altering drugs for combating the menace of communism. The CIA experimented with virtually every known psychotropic agent and that included ibogaine. Although most of this work was secret, some of the programs supported by MK-ULTRA involved funding individuals in academia who would carry out drug studies on “volunteers” and pass the information back to the CIA. One such individual was Dr. Harris Isbell, director of the NIMH addiction research center in Lexington, Kentucky. Isbell used ibogaine to treat eight African American men recovering from drug addiction. There was generally some interest at the time about the potential interactions between ibogaine and opioids, and the CIBA drug company had patented its use as a drug that would enhance the analgesic effects of morphine. Isbell’s experiments, which were conducted without patient consent, were looking for substances that could “rewire” the human brain and might positively impact recovery from opioid addiction and also be of use for the CIA’s secret brainwashing program. There can be no doubt as to the powerful psychotropic effects produced by ibogaine, particularly at higher doses, and in November of 1956 Isbell reported to the CIA that ibogaine was useful for treating heroin addiction. Unfortunately, his data never actually appeared in the public scientific literature.
In the 1960s, public experimentation with psychotropic drugs was becoming increasingly popular with young people in the USA and this included experimentation with ibogaine. Around 1962, a resident of New York city named Howard Lotsof , who was a heroin addict, began experimenting with ibogaine and other psychotropic agents which he hoped might be helpful in treating his addiction. Lotsof and 19 of his friends, seven of whom were heroin addicts, took a moderate dose of ibogaine. Most of them reported a reduction in their drug craving and withdrawal symptoms. Indeed, five of the seven reported that they were free from heroin use for up to six months.
Lotsof believed he had found the “cure” for opioid abuse disorders and began to proselytize for ibogaine’s use for the treatment of addiction. But several things stood in his way. First of all, he had no medical research credibility, that is no degree or other training that would help to convince people he could carry out a research program like this. Furthermore, in 1970 when ibogaine became a schedule 1 drug, it made performing research into its properties extremely difficult even for university-trained scientists. Thirdly, pharmaceutical companies weren’t interested in helping to develop ibogaine. Because ibogaine is a natural product, drug companies thought that they wouldn’t be able to get the sort of patent on it that they wanted if they were going to make a big profit. But Lotsof wasn’t easily put off. By the mid-1980s, he had managed to raise some private funding which allowed him to carry out informal trials with the drug in the Netherlands. A large number of informal but promising ibogaine trials treating drug addicts subsequently took place and, eventually, the NIH became interested in it as did an academic lab at the University of Miami. Unfortunately, all of this activity was cut short following the death of a patient in the Netherlands from a heart attack. Although it was never clear that ibogaine was responsible for this death, it naturally made people cautious. Informal testing of the drug continued in several countries around the world with a particularly large cohort of patients being treated in St. Kitts in the Caribbean. Other studies conducted in Brazil, New Zealand, and Mexico all suggested that ibogaine may have a significant beneficial effect when treating drug addiction. While most of these ibogaine studies have been fairly small, they have generally found that the drug seems to reduce cravings and physical withdrawal symptoms for individuals dealing with a variety of addictions, as originally observed by Lotsof.
Lotsof also made progress at the preclinical level. He persuaded Dr. Stanley Glick, a pharmacologist at Albany Medical College in New York to test ibogaine in morphine-dependent rats. The experiments appeared to work, reducing the animals’ desire to take morphine. Moreover, further experiments suggested that ibogaine was also effective in animal models of addiction to cocaine, alcohol and nicotine. The results were promising enough to encourage the Glick team to work with Dr. Martin Kuehne at the University of Vermont in an attempt to synthesize more potent, but hopefully less toxic, ibogaine analogs, leading to the synthesis of 18-methoxycoronaridine (18-MC). Like ibogaine, 18-MC also showed promise in animal studies of drug abuse. In order to promote ibogaine and related substances, Glick cofounded his own drug company, Savant Health and Wellness Partners. Through this organization, Glick was able to obtain an NIH research grant for just over $6.5 million that would take 18-MC through phase 1 human safety trials. Nevertheless, after working on 18-MC for nine years, Savant ran out of money and was not able to move the program to the next phase of clinical trials. In September 2019, MindMed bought 18-MC and also hired the team working on it. According to their publicity, MindMed now plans to run their own safety trials in the second half of 2020 and then hopefully proceed to the next stage of testing the effectiveness of the drug.
While all this is interesting, there are several things to note. The first is that ibogaine used at the doses previously required for treating addictions is not altogether free of side effects. Several people have died from cardiovascular issues over the years and, although it is not clear that ibogaine was responsible for these deaths, it is certainly quite possible. Ibogaine can also produce neurotoxic effects. Secondly, ibogaine produces profound hallucinations which are not really compatible with somebody wanting to take it on a routine basis and have a productive day at work. Which brings us to 18-MC. Because 18-MC produces some of the same beneficial effects as ibogaine in animal studies, if it turns out that 18-MC is also effective in treating human addicts but is non-hallucinogenic and doesn’t produce issues like heart problems, then it might be an excellent alternative to ibogaine itself. There are no published data on the effects of 18-MC in humans so far, although the CEO of MindMed has said that it looks to him as if the drug is free of hallucinogenic properties and is safe to take.
Nevertheless, we are still left with the biggest question of all, which is “how does ibogaine work?” This kind of knowledge is very helpful these days as drug regulatory organizations like to have an indication as to a drug’s mechanism of action if they are going to approve it for general use. Historically it has been thought that the profound psychotropic experience of taking ibogaine, as well as the ensuing drug-induced introspection, was key to helping an addict cope with a drug abuse issue. In other words, this was a transcendental type of cure based on profound introspection and self-analysis enabled by the drug. It was a mystical cure that was related to the original use of the drug by the Bwiti. But if 18-MC is not hallucinogenic—then what? We would have to conclude that the drug can produce effects on the nervous system that were beneficial for treating addiction and that these were not the same as the effects needed to produce hallucinogenic mystical and religious behaviors. Here we have a problem with ibogaine and drugs like 18-MC because really, nobody knows how they work. It’s not that they can’t be shown to produce effects in experiments that scientists carry out. In fact the situation is just the opposite. They produce every kind of effect but don’t produce any one of them very well—they are pharmacological dilettantes.
This is unusual. In the case of other types of hallucinogenic drugs, their mechanism is well established. For example, we know that classical hallucinogenic drugs like LSD, psilocybin, ayahuasca and mescaline all act as agonists at 5-HT2A receptors, a type of receptor for the neurotransmitter serotonin (5-HT). On the other hand, muscimol, the hallucinogenic molecule derived from the mushroom Amanita muscaria acts as an agonist at GABA-A receptors. Scopolamine and atropine, hallucinogenic molecules derived from a variety of plants, act as blockers of muscarinic acetylcholine receptors. However, none of these mechanisms appear to explain the effects of ibogaine. There is, however, another possibility. Some research has demonstrated that ibogaine’s major metabolite, noribogaine, is an agonist at κ-opioid receptors (KORs). Why might this be important? Here we should note the activity of another psychotropic natural product obtained from the plant Salvia divinorum, commonly known as magic mint. S. divinorum is used by the Mazatec Indians in Mexico to produce hallucinogenic effects in their religious ceremonies. The active molecule derived from the plant is called salvinorin A and seems to produce its effects by activating KORs. Indeed, other opioid drugs which activate KORs are also known to produce dysphoric hallucinatory effects. Ibogaine doesn’t activate KORs itself, so we might conclude that the psychotropic effects of ibogaine are mediated by its metabolite noribogaine. This would be a reasonable mechanism because quite a few drugs work this way. Heroin, for example, works through its conversion to morphine in the brain. Moreover, as 18-MC is free of KOR agonist activity, this would also explain why this drug is not hallucinogenic. Nevertheless, these explanations are very speculative.

However, if 18-MC is really not hallucinogenic then what explains the effects of ibogaine or 18-MC on substance abuse disorders? There are two types of explanations that have been put forward. The first of these is that, like other psychotropic drugs, there is a specific mechanism of action involved—such as an interaction with a neurotransmitter receptor or uptake system. On the other hand it has also been speculated that the effects of these drugs are genuinely non-specific and are the result of a mixture of low affinity sites of action. If there is a specific site of action, what could it be? One mechanism that has been suggested for the anti-addictive properties of ibogaine concerns its effects on nicotinic acetylcholine receptors in the brain. The important neurotransmitter acetylcholine works by activating two different types of receptors, nicotinic and muscarinic, and it is the former type that concerns us here. Because nicotinic receptors are constructed from five different protein subunits there are a very large number of different types that can be arranged by putting subunits together in different ways. Ibogaine has been shown to be an antagonist at several nicotinic acetylcholine receptor types including the α1β1 and α3β4 subtypes. The α3β4 receptor is expressed within the habenulo-interpeduncular cholinergic pathway of the brain, which seems to be involved in producing some of the rewarding effects of addictive drugs, and so it has been speculated that interaction with these receptors may be a major contributor to ibogaine’s beneficial effects on drug addiction. However, the reported effects of ibogaine on these nicotinic receptors are not all that potent, and those of 18-MC are even weaker, so this mechanism doesn’t seem to be all that convincing. Really, at this point in time, one would have to consider the hypothesis that the anti-addictive effects of ibogaine and 18-MC are produced by a large number of relatively weak effects on different neurotransmitter systems. Scientists don’t like conclusions like this—they like things to be clear cut. There are really very few drugs that are widely used today that don’t have a specific mechanism of action. Nevertheless, there is nothing in principle to suggest that a drug couldn’t produce useful effects in this way. Whatever the truth of the matter, ibogaine remains a scientific mystery and it will probably be necessary to keep returning to the question of its utility as a treatment for drug addiction and its mechanism of action again and again.
