When I emerged from viewing the current Blade Runner 2049 movie, I was forced to admit that things are not going well for the human race. The future seems bleak. According to the movie, Los Angeles in 2049 has lost its allure as a desirable place to live. In 2049 in Los Angeles it rains constantly. The air quality is so bad that you can hardly see a few feet in front of your face through the toxic sulfurous yellow fog (OK, so some things never change). Many people are reduced to living like animals. Of course, this isn’t the only vision of our possible dystopian future. The TV series Black Mirror presented many other versions. One of these, depicted in an episode entitled “Hated in the Nation”, described a future without bees. In this episode all the bees have disappeared because of some environmental disaster and have been replaced by mini-robot bees which, naturally enough, are being manipulated by evil people bent on world domination. It would certainly be sad if there were no bees around. Watching their fuzzy black and yellow bodies perched on a flower petal or buzzing as they fly through the warm air is a quintessential aspect of summer. It just wouldn’t be the same without bees. And there are other reasons why we should really appreciate bees. Bees are the most important pollinators of many flowers and crops something that is absolutely essential for human survival. Approximately 85% of all flowering plants are pollinated by bees, with honey bees (Apis Mellifera) and non-Apis bee species respectively pollinating $19 billion and $3 billions worth of crop plants in the United States alone (2010 data). And let’s not forget about honey and beeswax. Imagine a world without honey! Yes, bees are very important indeed.

Unfortunately, a bee apocalypse may really be in our not too distant future (Fig 1). For many years now there has been a gradual decline in the number of bees in all parts of the world. However, this slow decline changed dramatically in 2006 when there were many reports from the USA of what was described as “Colony Collapse”. This means that numerous colonies of bees simply disappeared. It wasn’t that the bees were just dying-no bee corpses were found as clues as to what was going on. It was just that they had gone-like a puff of smoke. But where had the bees gone and why? Naturally, there are a lot of theories about all of this. For example, it could be due to declining honey bee keeping practices, pathogens and parasites, loss of habitat or climate change. Although there is some evidence for all of these things, the most likely problem is the use of insecticides. Of course, the use of insecticides has revolutionized much of agriculture. The idea is to find substances that will kill insect “pests” but which do not harm humans. Generally speaking, several kinds of insecticides have been produced which do the job. But how about the different kinds of insects? Do insecticides do a good job of killing “bad” insects such as aphids and other agriculturally destructive plant-chewing, -piercing, and -sucking pests that devastate crops, but spare “good” insects like bees. Unfortunately, there is a strong possibility that insecticides are responsible for bee colony collapse. This idea has received a good deal of attention recently and there is an intense debate about the entire issue. In order to understand this debate, we first need to understand the nature of the insecticides and how they work.
The mostly widely used insecticides these days are known as neonicotinoids and, as one can see from their name, they were made to mimic the properties of the drug nicotine. So, what is nicotine and why might it be useful as an insecticide? The story of nicotine is obviously connected to the story of smoking tobacco which goes back to pre-Columbian times when plants of the genus Nicotiana were first cultivated. The Nicotiana are one of the largest groups of plants in the family known as the Nightshades (Solanaceae). Many of these species produce beautiful flowers and are widely used by gardeners throughout the world for their decorative effects. There are approximately 60 different species of Nicotiana distributed in the New World as well as Australia and Africa, although the vast majority of them are native to the Americas. Here they began as wildflowers growing primarily in the Andean highlands of Ecuador, Peru and Bolivia. With the advent of horticulture in South America the cultivation of Nicotiana mostly developed in the Northern parts of the continent around the Amazon-Orinoco basin and Guinea. The question is why this occurred? The most likely answer is that the tribes that cultivated Nicotiana did so because they had discovered the effects of nicotine, the psychotropic drug that is produced by these plants. Actually, as we shall see, nicotine and other similar molecules, don’t just occur in Nicotiana, but they are certainly found in highest amounts in species such as Nicotiana Rustica and Nicotiana Tabacum which were the main species cultivated by the indigenous peoples of South America.
It is said that the first person who actually smoked tobacco in Europe was a member of Columbus’ crew named Rodrigo de Jerez on his return from the New World where tobacco was first encountered by Europeans. However, the sight of him inhaling and exhaling smoke was immediately interpreted as evidence for some kind of Satanic possession and so he was thrown into jail for 3 years. Nevertheless, over the next few decades smoking became common on the Iberian Peninsula and in 1533 Diego Columbus, the son of Christopher Columbus, wrote that there were tobacco shops in many parts of Lisbon. However, the major initial attraction of the tobacco plant to Europeans was its decorative nature and it began to appear in gardens throughout Spain and France. It was also widely used in medicine. In 1559 Jean Nicot, a French diplomat serving in Lisbon, sent some seeds back to the court of the French queen Catherine de Medici with his own recommendation as to tobacco’s healing powers for diverse ailments. Catherine was very receptive to these ideas and immediately began growing the plants and using them in snuffs and poultices. Further positive reports as to the therapeutic properties of tobacco soon followed including a pamphlet entitled “Joyful News of Our Newe Founde Worlde” which reported that tobacco was extremely effective for treating virtually every disease known to man. The pamphlet was translated into most European languages and the use of tobacco spread rapidly throughout the continent, reaching the court of queen Elizabeth the first in London and even the Vatican.
Of course, today the most common method of using tobacco is smoking cigarettes, something which had its origins in nineteenth century Spain. Here there were plenty of tobacco factories where young ladies like Bizet’s Carmen were employed to hand role tobacco leaves into cigars. The girls would keep any remnants for themselves, shred them and role them up in papers to smoke as papelottes, something that became popular with the poorer classes throughout Spain. The habit was picked up by Napoleon’s troops returning from the Iberian Peninsula to France where they also became popular and were referred to as cigarettes. As with Carmen in Spain, the cigarette in France was initially associated with loose women. Generally speaking, cigarettes were thought of as being effeminate as opposed to the manly habit of smoking pipes or cigars. Nevertheless, by around 1840 cigarettes were being smoked widely by all classes of people in Paris. The cigarette was certainly very portable and, after the invention of “Lucifers” (friction matches), they were easy to light as well.
Although tobacco was used recreationally for smoking, we know that starting in Nicot’s time, it was also used as a medicine. Different preparations of tobacco such as poultices were rubbed on the skin and extracts of the plant were ingested for many different purposes. There were early attempts to concentrate and purify the chemical(s) that were ultimately responsible for the effects of tobacco. Initial attempts to do this took place in the 17th century through the distillation of tobacco from which a potent oil could could be obtained. This “Oil of Tobacco” was used in the treatment of skin diseases but was also found to be extremely poisonous when ingested. It wasn’t until the 19th century that the science of organic chemistry reached a degree of sophistication that enabled the active substance responsible for the effects of tobacco to be completely purified. This was achieved in 1828 by Posselt and Reiman. The new substance was duly named nicotine as it was isolated from Nicotiana Tabacum, thereby indirectly recognizing the contribution of Jean Nicot to the history of tobacco use. The chemical formula of nicotine was elucidated in 1843 and its molecular structure in 1893.The isolation of nicotine in the early 19th century should be put into context of other remarkable advances in the chemistry of natural products at the time. In 1805 the young German pharmacist Friedrich Serturner had isolated morphine, the major active component of crude opium. Soon after this many other important substances were isolated from plants. These included a second substance isolated from opium named codeine, as well as caffeine, atropine, strychnine and quinine to mention just a few. Because these molecules were found to have a basic or alkaline quality they were named “alkaloids”, a term that is now in general use for describing natural products of this type. As we shall discuss further, some of these alkaloids including coniine from poison hemlock (Conium maculatum) and lobeline from lobelia (Lobelia tupa) turned out the have chemical structures and biological properties, including profound toxicity, which were extremely similar to nicotine.
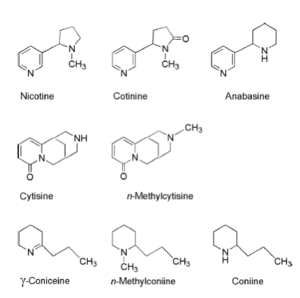
The toxic nature of nicotine and other nicotine like natural products derived from plants is a story which has developed in parallel with the history of the smoking of tobacco. The earliest tale, and still the most famous, concerns the death of the great philosopher Socrates in 399 BC. The details of his death come to us from the writings of his pupil Plato, particularly from his dialogue known as the Phaedo. Socrates was found guilty of corrupting the youth of Athens and sentenced to death. He was made to drink an extract of the poison hemlock plant. There is a description of the symptoms of his death-a creeping numbness that started in his feet and traveled up his legs until it reached his heart which then stopped. In fact, it is not only hemlock but several other plants that contain nicotine-like molecules (Fig. 2). Hemlock, as mentioned above, contains coniine. Indeed, throughout history the hemlock plant has been famous for its poisonous effects and has been used as a criminal device as reflected in many of the stories of Agatha Christie and other crime writers. The laburnum plant (Laburnum anagyroides) contains the nicotine like substance cytisine and the blue cohosh (Caulophyllum thalictroides) contains N-methyl-cytisine. Many other similar substances occurring in minor amounts are also found in these and other plants. While there are few instances these days of humans dying from the effects of inadvertently consuming plants that contain nicotine like alkaloids, more frequent problems exist with cattle who eat such plants while foraging. However, there still are occasions when humans can be exposed to harmful concentrations of nicotine. The most common of these is Green Tobacco Sickness. When cultivated tobacco plants get wet from rain or dew, some of the nicotine is leached out of the plant on to the surface of their leaves. When workers walk through the tobacco fields they rub against these wet surfaces transferring some of the dissolved nicotine on to their skin from which it is then easily absorbed into their circulation. As it turns out a tobacco worker can accumulate an appreciable amount of nicotine in this way while working in the fields for several hours. Accumulation of high doses of nicotine produces a wide variety of symptoms including weakness, headache, nausea, vomiting, dizziness, abdominal cramps, breathing difficulties, abnormal temperature, pallor, diarrhea, chills, fluctuations in blood pressure or heart rate and increased perspiration and salivation. As there are some 33 million tobacco workers in the world today harvesting tobacco in a large number of countries, this syndrome is fairly common unless careful precautions are taken such as wearing garments with long sleeves. As we shall see the toxic effects of nicotine and similar substances are also the basis of their widespread use as some of the world’s most effective insecticides.
We are therefore left with a somewhat confusing picture of nicotine. On the one hand it’s a recreational drug used by billions of people. Lots of people clearly like smoking cigarettes. But it’s complicated. The nicotine that accompanies a cigarette produces positive effects but its also very addictive. On the other hand, in larger amounts, nicotine is a highly toxic substance that can be responsible of the deaths of humans and animals. How can we reconcile these two quite different impressions of this one substance? To do this, we must first understand exactly how the drug works.
In order to communicate with one another, or with tissues such as muscle, nerve cells secrete chemical substances called neurotransmitters which traverse a tiny gap called a synapse between the nerve and the cell it contacts. Here the neurotransmitter binds to a molecule called a receptor and activates it-rather like a key fitting into a lock and opening a door. Activation of receptors changes the state of target cells increasing or decreasing their ability to generate electrical signals called action potentials. There are numerous different types of neurotransmitter molecules as well as many types of receptors. However, the first neurotransmitter to be discovered, and certainly one of the most important, is called acetylcholine. The receptors for acetylcholine are of two types –nicotinic and muscarinic. In the first case, the activity of acetylcholine on its receptor is mimicked by nicotine and in the latter case the receptors for acetylcholine can be activated by the drug muscarine. Actually, the way nicotinic and muscarinic receptors work is quite different. Here we will only be concerned with nicotinic receptors.
Nicotinic acetylcholine receptors are very widely expressed both in the central nervous system (brain and spinal cord) and in the peripheral nervous system (the somatic and autonomic nervous systems). In the central nervous system, nerves generally make connections with one another but in the periphery they also make connections with muscles and glands. The activation of nicotinic receptors in the brain by acetylcholine has a number of important roles, particularly the regulation of several phenomena that are generally termed “cognitive functions”. In the peripheral nervous system somatic nerves that utilize acetylcholine innervate many of our muscles where they activate nicotinic receptors and make muscles contract. In the autonomic nervous system nerves usually pass through a way station called an autonomic ganglion before continuing on to their final destinations. The nerves that innervate these autonomic ganglia are also cholinergic and activate nicotinic receptors within the ganglia.
When acetylcholine, or a drug like nicotine, activates a nicotinic receptor it usually has an excitatory effect. By this one means that it encourages the target nerve or cell to fire an action potential. The way it does this is to “depolarize” the target cell, bringing it closer to the threshold for firing an action potential. How does activation of a nicotinic receptor produce depolarization? The nicotinic receptor is actually a complex of protein molecules that act as an ion channel. Normally the channel is closed, but when it is activated by acetylcholine it opens briefly and allows positively charged sodium ions to pour into the cell and positively charged potassium ions to pour out of the cell. The movement of these ions is what is responsible for the observed depolarization and the reduction of the electrical potential difference across the cell membrane.
Finally, we need to answer one last question. What is the precise structure of the nicotinic receptor that allows it to function as an ion channel and to be activated by acetylcholine? The answer is that the receptor is made up of five protein molecules or “subunits” (Fig. 3). These are arranged like the staves of a barrel with a hole down the middle. Normally the hole is occluded but when acetylcholine interacts with the proteins that make up the channel they change their shape slightly, dilating the hole at the center of the channel so that it is fully opened and sodium and potassium ions can flow through it until it closes again.
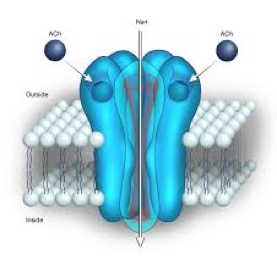
The proteins that are used for constructing nicotinic receptors throughout the body are very similar and so all nicotinic receptors work more or less the same way wherever they are found. However, there are some small differences in the structures of the proteins in different parts of the nervous system, resulting in receptors that have unique properties. Sometimes we find that nature has provided us with natural products, such as molecules found in some snake venoms, which can target a particular type of nicotinic receptor and block it. This is one way in which animals like snakes can paralyze their prey. These molecules are very useful to scientists performing studies where they wish to identify a specific nicotinic receptor subtype with a particular molecular structure.
Do insects have nicotinic receptors? The answer is that they do. Acetylcholine acting at nicotinic receptors has many important functions in the nervous system of insects. One would therefore imagine that applying drugs like nicotine to insects might well be toxic to them just as it can be to humans. This was first realized several hundred years ago. Tobacco was first used as an insecticide in 1690, a soluble extract of tobacco being applied to infested pear trees in France. Tobacco water and tobacco powder were recommended in 1763 as a remedy for plant lice in France and were used successfully in 1773 against aphids and red spiders in England. By 1800 tobacco was in common use as an insecticide in England. For example, tobacco dust could be blown from a device similar to a hair dryer on to aphid infested trees and infested leaves were dipped into a strong tobacco solution. The first recorded use of tobacco as an insecticide in America was in Albany in 1814, when tobacco water was used for getting rid of aphids. In the same year, a force pump was employed to squirt tobacco water onto caterpillars in England. Tobacco extracts were also used as fumigant insecticides in which nicotine containing smoke was spread across crops or orchards. In 1773 tobacco was put into an iron pipe which was heated and the smoke from it was blown onto infested plants by the use of a bellows. In 1800 it was reported that a pair of bellows was used to force smoke under a tent which had been put over nut trees infested with aphids. In 1828 a tent on wheels was put over a grapevine trellis and in 1839 growers were advised to burn paper saturated with tobacco extract under a tent stretched over peach and nectarine trees to kill aphids. As the Industrial Revolution advanced so did the technology for the use of insecticides. In 1851 a fumigator which burned tobacco and ejected the smoke was invented and put over rose bushes infested with aphids.
Although this list of observations clearly attests to the effectiveness of nicotine as an insecticide, its use declined in modern times owing to the growing realization that the drug also produces toxic effects on humans and animals who could inadvertently be poisoned by nicotine if it was sprayed over large areas. It was realized that what would be of greater utility would be a similar type of insecticide that would act upon the nicotinic receptors found in insects but not those found in man. Investigations as to the properties of nicotinic receptors in insects showed that they did function in a similar manner to mammalian receptors but there were also enough differences from the structural and functional points of view to suggest that it might by possible to produce synthetic molecules that specifically targeted insect receptors. This idea began to bear fruit in the late 1970s with the synthesis of the first of the group of molecules called neonicotinoids which proved to be highly effective insecticides. Imidacloprid (Fig 3), the first of the neonicotinoid class of insecticides, was patented in 1985 by the Bayer company and was first marketed in 1991. It exhibits strong insecticidal activity against really problematic pests such as aphids. Several studies demonstrated the action of these molecules at insect rather than vertebrate nicotinic receptors resulting in their selective action on insects. The neonicotinoid pesticides proved to be a great success and became very widely used, and they still are. However, their absolute utility has come under some scrutiny. As things turn out it appears that the neonicotinoids do harm bees as well as insect pests. Hence, there have been moves to restrict the use of neonicotinoids in both the United States and Europe.
How exactly do neonicotinoids affect bees? One of the reasons why nicotine is so toxic to animals like humans is because nicotinic receptors mediate neurotransmission at the neuromuscular junctions that control the functions of skeletal muscles. Interference with this process can lead to paralysis and death. However, neuromuscular transmission in bees isn’t controlled by acetylcholine but by a different neurotransmitter called glutamate. So, neonicotinoids don’t paralyze bees. Rather the nicotinic receptors in bees are all localized to their brains. As it turns out bees have quite a sophisticated repertoire of “beehaviors” and rely on their memories to perform important tasks such as finding the appropriate flowers to visit. Individual bees can even be trained to carry out specific tasks in a laboratory. Drugs that activate and desensitize (inhibit) bee nicotinic receptors in their brains can be shown to profoundly disrupt their behaviors. For example, neonicotinoid treated bees have a hard time remembering things. It should be noted that although the structure of bee nicotinic receptors resembles those found in humans, there are quite a few differences in the amino acids that make up the various protein subunits (bees have 11 of them) in the two species. Consequently, although both bee and human nicotinic receptors can be activated by acetylcholine and nicotine, only bee receptors are sensitive to neonicotinoids.
However, it is all very well to demonstrate that neonicotinoids can have effects on bees in a laboratory setting. One argument is that these effects are only apparent when using high drug concentrations that are not actually encountered by bees in nature. Neonicotinoids are taken up by plants and transported to all their organs, including flowers, thus contaminating pollen and nectar as well as any fluid produced by the plant. When bees visit flowers they drink nectar and remove pollen, and neonicotinoids have been found to contaminate both of these. But are the concentrations of insecticides high enough to really disrupt bee behaviors? Recently, a paper was published in the prestigious journal Science that helps to answer this question. Mitchell et al (1) collected samples of honey from 198 different samples scattered on every continent with the exception of Antarctica. As the authors point out, individual bees usually collect nectar and pollen in an area limited to 4 km from the hive, but may travel up to 12.5 km away under some circumstances, which makes bees effective “sentinels of environment quality”. The authors then examined every honey sample for the presence of several neonicotinoids including acetamiprid, clothianidin, imidacloprid, thiacloprid,and thiamethoxam (Fig 4).
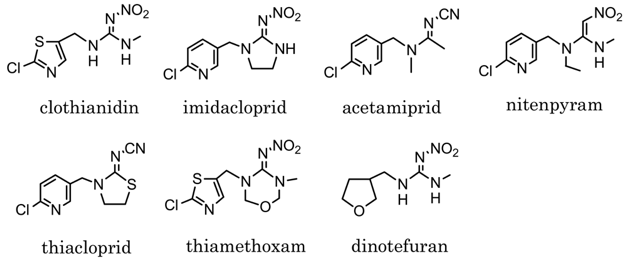
Their results demonstrated that “75% of all honey samples contained quantifiable amounts of at least one neonicotinoid.” This proportion varied considerably among regions, being highest in North American (86%), Asian (80%), and European (79%) samples and lowest in South American samples (57%). Thirty percent of all samples contained a single neonicotinoid, 45% contained between two and five, and 10% contained four or five. As for the significance of these findings, quoting the authors again “The total concentration of the five measured neonicotinoids was, on average, 1.8 ng/g in positive (i.e. contaminated) samples and reached a maximum of 56 ng/g over all positive samples. This average concentration lies within the bioactive range causing deficits in learning, behavior, and colony performances in honey bees.” Indeed, it has been shown that several of these compounds produce additive effects on bee behavior, meaning that in some cases the total effects of finding several neonicotinoids in a bee population might be quite significant. Given these results the bans that have been imposed on current neonicotinoid use might be seen as appropriate or even perhaps too little too late. It is possible however that current neonicotinoids could be further improved in the future. The structures of the nicotinic receptors in the brains of different insects are different enough to encourage the belief that agents could be produced that were selective for particular species and so have minimal effects on bees and other important insects.
Although the reported situation with respect to bees is certainly serious it may only be the tip of the iceberg. A second recent paper (2) in the journal PLoS One has reported that in just 3 decades, insect populations in Germany have declined by more than 75%. The authors of the paper noted that in recent years, there had been a steep decline (82%) in the total number of insects, known as the “biomass”, caught in their traps during the summer season when insect populations peak. The magnitude of these declines is really catastrophic. Insects are an important part of our environment and have many vital roles to play. Wiping them out would be a disaster. The reason for the decline is unclear but insecticides are certainly one of the factors suspected.
The current series of reports on the state of the world’s insect population, together with those on other endangered species, are likely to be the harbingers of an ever more dystopian future if we don’t do something about it soon. Los Angeles 2049 is not that far away.
1) Mitchell EAD et al (2017) A worldwide survey of neonicotinoids in honey. Science. 358:109-111
2) Hallmann CA et al (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS One. Oct 18;12(10):e0185809. doi: 10.1371/journal.pone.0185809. eCollection 2017.
