There has been some welcome news in the last few weeks about the COVID-19 pandemic: it looks like the hunt for a vaccine that could be used to protect people against the disease may have been successful. And not just one vaccine. There have been several announcements detailing the outcomes of clinical trials, the gold standard for determining the therapeutic effectiveness of proposed treatments, reporting extremely promising results for four vaccines—and, it seems, several more good bets are in the pipeline. If indeed these vaccines ultimately become widely used, it would represent a considerable achievement because vaccines usually take many years to produce from “scratch” but, in these cases, success has apparently been achieved within a year. This is certainly at “warp speed” or even faster, raising the question as to why vaccines previously took so long to produce and what will happen in the future?
In Western countries, the practice of inoculation developed in the context of smallpox (Variola) infection, a disease we now know is caused by a virus. Smallpox used to be one of the most feared and deadly diseases killing, or permanently disfiguring, billions of people over the years. There is plenty of evidence for smallpox infections dating at least as far back as ancient Egypt. The disease was eventually spread throughout the rest of the world by European colonists to the indigenous peoples of the Americas and Australasia, where it routinely produced devastating effects on populations who had no historical immunity to it. Smallpox killed everybody; it certainly didn’t discriminate between races or classes as exemplified by the deaths of Louis XV of France in 1774 and Queen Mary of England in 1694.
Although there was no clear idea as to how a disease like smallpox was spread, it was obviously contagious and it was widely recognized that individuals who caught it and then recovered were unlikely to catch it again. Indeed, during the Middle Ages, some parents would try to have their children catch the disease on purpose thinking that a mild dose suffered as a child might be protective in adulthood. In 1700, a paper was read to the Royal Society in London describing the Chinese practice of inhaling dried smallpox scabs to try and catch the disease and produce subsequent immunity; another paper, read in 1713, described the Turkish practice of inoculating people with material from smallpox pustules. This practice came to the notice of Lady Mary Wortley Montague, the wife of the British ambassador in Constantinople between 1716 and 1718, who wrote, “The Small Pox, so fatal and so general amongst us, is here entirely harmless by the invention of engrafting”—certainly a striking observation, and she became determined to introduce the practice into England once she returned, in spite of considerable resistance to the idea. Nevertheless, Lady Mary persevered and she eventually persuaded a doctor to inoculate her 3 year old daughter, who then survived a raging smallpox epidemic. This was the first example of the use of inoculation to be recorded in the British Isles or Europe. The Rubicon had been crossed and gradually more and more people were treated. The King’s doctor, Sir Hans Sloane, conducted a “clinical trial” on inmates in the Newgate prison—who all survived. Eventually even members of the Royal family were inoculated and the process, known as “variolation” (from Variola) began to be much more widely accepted. Some people even made a profession out of traveling around the country and expertly variolating people for a fee.
Of course, treating a disease in this way always came with the possibility of actually contracting a serious case of smallpox. A huge advance was made in the late 18th century by the English doctor Edward Jenner. Jenner followed up stories he had heard that milkmaids who contracted the relatively mild disease cowpox from animals that they cared for also became resistant to smallpox—cowpox being a very minor problem in humans. He therefore began inoculating people with cowpox-derived matter and observed that they also became resistant to smallpox just as effectively, although with much less accompanying risk. Because the material he used came from cows (Vacca is Latin for a cow), this procedure became known as vaccination. Eventually, at the suggestion of Louis Pasteur, the term vaccination was broadened to include all procedures of this type.
Although nobody knew at the time why variolation or vaccination was effective, we now know that it is due to the activation of an immune response against the infectious pathogen. The adaptive immune response recognizes biochemical features on infectious organisms and then counters them by producing neutralizing antibodies (humoral immunity) or specialized immune cells (T-cells) that destroy pathogen-infected cells. A vaccination “trains” the immune system to produce these specific responses by introducing some version of the infectious agent so that when a real infection comes along, it already has the requisite arsenal of antibodies and T-cells to neutralize it before the infection can take hold. Jenner’s breakthrough demonstrated that as long as the inoculated material could evoke the proper immune response, by virtue of the fact that it was recognized in the same way as the pathogen, it didn’t have to be the disease itself, opening the door to the design of safer effective vaccines.
This approach bore fruit in the future, as in the polio virus scare in the 1950s when Jonas Salk pioneered the use of a polio vaccine based on an inactivated polio virus. Even at this late date, there was still considerable skepticism about whether an inactivated virus of this type, which couldn’t cause any form of the disease, would be able to produce really long-lasting immunity, and so a parallel effort producing a vaccine from live viruses was also undertaken by Albert Sabin and his colleagues. In the end, both vaccines were successful, having different advantages—and both were used to great effect.
Nowadays, the use of contemporary molecular biological techniques has resulted in methods for making vaccines that are a considerable advance on the methods that Salk and Sabin used. Generally speaking, it is these newer methods that have been used to make vaccines against SARS-CoV-2, the virus responsible for the COVID-19 syndrome. To understand these new methods, we should first examine the pathogenic mechanisms that allow the SARS-CoV-2 virus to infect cells. Coronaviruses like SARS-CoV-2 commonly produce infections leading to respiratory, enteric and central nervous system symptoms in humans and other mammals. In fact, animal viruses sometimes mutate and then “jump” to humans. Such “zoonotic” origins are likely to be the source of SARS-CoV-2 and also of similar coronaviruses that have produced serious human infections in recent years. Notably, a bat coronavirus named BatCoV-RaTG13 has been identified as being the most closely related virus to SARS-CoV-2 on a genomic level, exhibiting a 96% sequence identity although, to date, the direct precursor of SARS-CoV-2 and the involvement of an intermediate animal host in the emergence of the human virus remains to be unequivocally determined. At the beginning of the twenty-first century, two coronaviruses, SARS-CoV and MERS-CoV were discovered that resulted in serious respiratory infections in humans (SARS stands for Serious Acute Respiratory Syndrome; MERS for Middle East Respiratory Syndrome), although both of these epidemics were soon contained. Then, in December 2019, a novel coronavirus (SARS-CoV-2) emerged in Wuhan, Hubei, China, and it is this virus that is responsible for the current COVID-19 pandemic.
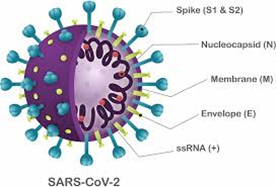
The genetic material of the SARS-CoV-2 virus is a positive sense single-stranded RNA molecule. The virus propagates itself by inserting this genetic information into target human cells. The RNA then acts as a source of instructions for the cells to produce further viruses. In order for viral entry to occur, the virus must first attach itself securely to a target host cell. The attachment of SARS-CoV-2 to a host cell is initiated by its spike (“S”) protein trimer, which sticks out of the membrane surrounding the virus, making it look like a crown, and this is the source of its name. The S-protein is a class I fusion glycoprotein, analogous to proteins that have similar functions on other viruses such as the influenza hemagglutinin (HA), the respiratory syncytial virus (RSV) fusion glycoprotein, and the human immunodeficiency virus (HIV) gp160 envelope protein. Given the importance of the S-protein in viral pathogenesis, it was quickly realized that it might be a good target for neutralizing antibodies that might counter viral infection.
The S-protein binds to target cells that express the protein angiotensin converting enzyme 2 (ACE2) on their surface. The rod-like spike proteins on the surface of SARS-CoV-2 are the tip of the spear that drives COVID-19 infection. When the protein spikes of the virus bind to human cells via ACE2, they are cleaved by a proteolytic enzyme and then dramatically change shape. They jack-knife, folding in on themselves to fuse their own membrane with the membrane of host cells. This opens the door to coronavirus infection.
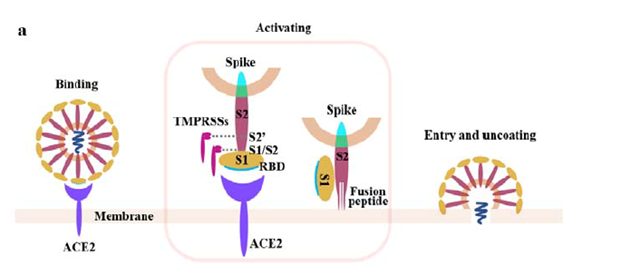
(from bioRxiv preprint doi: https://doi.org/10.1101/2020.02.08.926006)
Preclinical research quickly demonstrated that antibodies that bound to the S- protein, and to its receptor-binding domain (RBD) in particular, prevented its attachment to host cells and its subsequent entry into these cells. On the basis of this knowledge, and information that had been previously gained when attempting to make vaccines for treating SARS-CoV and MERS-CoV, it seemed very likely that a vaccine that targeted the SARS-CoV-2 S-protein would be similarly effective.
According to the current model, vaccines are being prepared “at warp speed” which has meant making innovations that allow the testing and manufacture of new candidate vaccines to occur very quickly, contracting the traditional time for vaccine development which usually lasts at least 5 years and can take considerably longer. This has been achieved by skipping many of the normal safety trials, often performed using animals, or at least running them in parallel with the normal clinical trials which involve humans. A document released by the International Coalition of Medicines Regulatory Authorities (ICMRA) at the start of the pandemic stated the following:
- For all SARS-CoV-2 vaccine candidates it is necessary to obtain data in animals and to characterize the immune response induced by a SARS-CoV-2 vaccine candidate.
- It is not required to demonstrate the efficacy of the SARS-CoV-2 vaccine candidate in animal challenge models prior to proceeding to FIH clinical trials.
Based on preclinical experiments with SARS-CoV-2, as well as previous experience with SARS and MERS, the question then became how best to enable humans to produce neutralizing antibodies against the SARS-CoV-2 S-protein? There are really two main approaches to this problem. One is to make a vaccine the traditional way; like the original polio vaccines, one could use live or inactivated SARS-CoV-2 viruses to provoke an immune response as the basis of a vaccine. In this case, “live” means a virus that can replicate in the human host but has been “weakened” by having its genes modified so that it doesn’t replicate as well as the original virus. Nevertheless, there is always the chance that it will back mutate and produce fully fledged disease in some individuals. In addition, because nowadays we have much more sophisticated biochemical techniques at our disposal, one can make the purified S-protein itself and use this material as the basis of the vaccine. Several vaccines that are based on these methodologies are currently in the pipeline. For example, Chinese vaccine makers have developed several vaccines using traditional methods that are in the final stages of testing, although none of them have yet completed the Phase 3 trials that are considered the gold standard for demonstrating vaccine effectiveness and safety. Nevertheless, some of these vaccines have already been used under policies known as emergency use authorization in China as a source of protection for frontline health workers.
However, the current excitement about vaccines against SARS-CoV-2 really revolves around newer technologies that involve basing the vaccine on genetic material that codes for the S-protein rather than the SARS-CoV-2 virus itself or proteins purified from it. The idea here is that patients are injected with the RNA or DNA that encodes the S-protein in a manner that will allow the nucleic acid to be taken up by host cells. The host cells will then use the genetic material as a set of instructions for making the S-protein and displaying it on host cell membranes. The host’s adaptive immune system will then recognize the S-protein and engage with it, producing an immune response consisting of neutralizing antibodies and T-cells. The most straightforward way of doing this is just to make mRNA that codes for the S-protein, encase it in suitable lipid vesicles and inject it into the patient. It has been demonstrated that lipid vesicles of the type used in these vaccines are efficiently taken up into cells by the process of endocytosis. Although this procedure seems like a really good idea, no commercial vaccine has ever been produced this way. A slightly more complicated approach is to make a “vector”—usually a different sort of inactivated (replication-incompetent) or weakened (replication-competent) virus such as an adenovirus—and insert the gene coding for the S-protein into its genome. Upon injection, these virus particles will infect host cells which will then start to synthesize the S-protein as part of the viral genome and provoke the appropriate immune response. These are the two methods that have been used to create the new vaccines that have recently been in the news.
The first promising news to be released was from the giant pharmaceutical company Pfizer, working in collaboration with a German biotech company called BioNTech. It was reported that in advanced human trials (Phase 3), they had tested a new vaccine that was around 95% effective, which is extremely good in comparison to many other vaccines that are used routinely—flu vaccines, for example, are usually around 60% effective. This vaccine, named BNT162b2, had previously been reported to exhibit promising properties in preclinical trials where it protected rhesus macaque monkeys from infection by SARS-CoV-2. In the BNT162b2 vaccine, lipid microvesicles contain the RNA for the S-protein modified by a few small changes. First of all, some of the RNA contains an unnatural nucleoside, N1-methyl-pseudouridine (m1Ψ), that helps it to avoid being recognized by the host’s innate immune system, which might otherwise block its effects. Furthermore, an enzyme cleavage site that normally helps the S-protein to change shape as a prelude to target cell fusion was modified in order to keep the protein in a more “immunogenic” conformation. Analysis of the data from the Phase 3 clinical trial, in which patients received two inoculations with the vaccine over a period of several weeks, showed that it conferred protection against naturally occurring SARS-CoV-2 infection by 95%. This efficacy was consistent across age, gender, race and ethnicity demographics. The observed efficacy in adults over 65 years of age was over 94%.
Very similar results were reported at almost exactly the same time by the biotech company Moderna, working together with the US government. Like the Pfizer/BioNTech vaccine, the mRNA-1273 vaccine candidate manufactured by Moderna, employed RNA that encoded a conformationally stabilized S-protein encased in lipid microvesicles. Both of the two RNA-based vaccines were very similar in their overall composition, and the preliminary Phase 3 results were very promising—the Moderna vaccine also providing around 95% protection. Further results will be released in due course. If these vaccines are ultimately successful, they will represent the first time that RNA technology has been successfully employed in a widely distributed vaccine for human use. Are there any real differences between the two vaccines, as they both work by precisely the same principle? As mRNA can quickly degrade in warm temperatures, vaccine storage presents a serious practical challenge. While Pfizer’s vaccine must be stored at –70 °C, Moderna’s can be kept at –20 °C. Moderna also noted that once thawed, its vaccine can remain stable for a month at 2–8 °C.
In addition to the two RNA viruses, other promising candidates were also announced. One of these has been produced in the UK by the pharmaceutical company AstraZeneca in collaboration with Oxford University. The Oxford vaccine (ChAdOx1 nCoV-19) has been made from a weakened version of a common cold virus (adenovirus), that was genetically altered so that it is impossible for it to grow in humans. The adenoviral genome was modified to contain the gene for the wild type (non-stabilized) S-protein. Like the Pfizer and Moderna vaccines, the Oxford vaccine had previously undergone successful testing using monkeys (macaques), and ,in humans, it was reported to produce either 62% or 90% protection depending on how one interprets the data. Adenovirus vaccines have been extensively researched and widely used for decades and have the significant benefit in that they are stable, easily manufactured, transported and stored at domestic fridge temperature (2–8 °C). This means they can be easily distributed using existing medical facilities such as doctors’ offices and local pharmacies, allowing for the vaccine, if approved, to be deployed very rapidly. Another successful adenovirus-based vaccine has been produced in Russia under the name “Sputnik,” which seems very reasonable owing to the very virus-like shape of that legendary satellite. This vaccine, which consists of a combination of two adenoviruses, was also highly protective in humans. Trials on two further adenovirus-based vaccines are reported to be near completion—one from a Chinese company and another from the US pharmaceutical company Johnson and Johnson. Although all of this is good news, there are still a number of things that need to be clarified. Primarily, how long does immunity last? Are people of all age groups adequately protected: is the vaccine effective in the pediatric population? Can a vaccine be made that is stable and easy to administer, for example by the intranasal route?

In addition to the arrival of all these new vaccines, there is another related approach for neutralizing SARS-CoV-2, which is known as passive immunization. The basis of this approach is to take blood from people who have recovered from COVID-19 and who are now immune to SARS-CoV-2 infection by virtue of the fact that they have made antibodies against it. The idea is to find the population of antibodies in the blood of recovered patients that is best at neutralizing the virus, expand it, and then inject it into a host who will also become immune. As opposed to a vaccine where the host constantly makes his/her own antibodies, with passive immunization somebody else has made them and they are all ready to go ,so that they can be rapidly effective in very sick patients, as opposed to vaccination which takes several weeks to achieve immunity. This method has been shown to work in some instances as a way of producing immunity but, unlike vaccination, the conferred immunity is short term in nature. Convalescent plasma also has several limitations, including batch-to-batch variability and a requirement for blood-type matching. Samples must also be screened for blood borne pathogens, including hepatitis viruses, HIV and parasites. There are also potentially other ways of preparing suitable antibodies which might be employed for passive immunization. These types of approaches have been used for prepare neutralizing anti-SARS-CoV-2 antibodies. Recently, the FDA granted emergency use authorization for Eli Lilly’s neutralizing antibody bamlanivimab (LY-CoV555). LY-CoV555, is a potent anti-spike neutralizing antibody prepared from a convalescent COVID-19 patient. Biochemical, structural, and functional characterization of the antibody revealed high-affinity interactions with the ACE2 receptor-binding domain of the S-protein and potent neutralizing activity in a rhesus macaque model where the animals were challenged by injection of SARS-CoV-2. Bamlanivimab is going to be used for the treatment of mild to moderate COVID-19 in adults and pediatric patients 12 years and older with a positive COVID-19 test who are at high risk for progressing to severe COVID-19 and/or hospitalization. Regeneron has also announced their antibody “cocktail” therapy (REGN-COV2) combination consisting of casirivimab (formerly REGN10933) and imdevimab (formerly REGN10987). Both are neutralizing monoclonal antibodies designed to bind non-competitively to the receptor binding domain of SARS-CoV-2’s spike protein by targeting non-overlapping sites. Excitingly, on 2 October 2020, Regeneron announced that US President Donald Trump had received “a single dose of REGN-COV2 after testing positively for SARS-CoV-2.” The preparation was provided by the company in response to a “compassionate use” (temporary authorization for use) request from the president’s physicians. Perhaps this is why the president recovered from COVID-19 so rapidly?
All of these results suggest very strongly that an effective vaccine will be with us sooner rather than later. Moreover, a great deal has been learned about vaccine production which should allow subsequent vaccines to be produced much faster than previously. Now, of course, people will have to be vaccinated en masse. The recent news that 4 out of every 10 US citizens do not want to be vaccinated against SARS-CoV-2 because of misinformation is very discouraging, and it is unlikely that scientists by themselves can put this right. It’s not just about science. Government leadership is also urgently required for this program to be fully effective.
