One afternoon in the autumn of 1969 I visited Lewis’ medical bookstore on Gower St in London, just a few blocks away from University College. I had been doing so for several years because Lewis’ always displayed copies of the scientific journal Nature for the public to read or purchase. While at the bookstore that afternoon I read what I consider to be the most remarkable scientific paper I have ever encountered (1). The subject of the paper was the hallucinogenic or “psychedelic” drug mescaline. I had just returned from spending the spring and summer working and traveling around the USA prior to attending university to study biochemistry, and I wanted to catch up on the latest scientific news. My travels in the USA had included visiting San Francisco and also attending the famous Woodstock rock festival in upstate New York. Needless to say, I was overflowing with enthusiasm for hippie culture and the associated music and psychedelic drug taking. In fact, I had become interested in psychedelic drugs prior to that time while I was still at high school in the late 1960s. I had read Aldous Huxley’s books “The Doors of Perception” and “Heaven and Hell” and was fascinated by his descriptions of the effects of the drug mescaline. I even tried to make some of it in the school laboratory but didn’t get very far. Surely, I thought, if we could understand how this drug worked it would lead to a better understanding of the phenomenon of human consciousness.
There was a theory circulating at that time that certain people produced an abnormal endogenous psychedelic molecule which elicited the symptoms of schizophrenia. Indeed, many people thought that the effects of hallucinogenic drugs closely resembled schizophrenic behavior. The abnormal molecule could be detected in the urine of schizophrenic patients as a chromatographic pink spot. Theories such as these were known as “Orthomolecular Psychiatry”—the idea that diseases like schizophrenia were caused by abnormal chemical factors, not by social factors like interactions with one’s parents. Hence, if one treated patients with the correct chemical compounds, you could prevent them making their own “psychotogens” and cure the disease. At the time, this seemed to me to be an entirely reasonable idea. One day I read a small advertisement in the Times newspaper saying that a Mrs. Gwynneth Hemmings was going to found a society to investigate Orthomolecular Psychiatry. People who were interested were invited to attend an organizational meeting at a church in Kensington. I decided to go along and became a founding member of the new British Association for Schizophrenia. At the meeting, there was considerable discussion about the pink spot and what it could mean. The chemical structure of the pink spot was supposed to be 3,4 dimethoxyphenylethylamine (DMPEA), which was very close to that of mescaline (3,4,5 -trimethoxyphenylethylamine). Even though synthetic DMPEA wasn’t able to reproduce the psychedelic effects of mescaline, it was thought that it might be derived from another molecule produced by schizophrenics which was hallucinogenic but hadn’t yet been identified. It seemed that understanding the drug mescaline could be a key to understanding diseases like schizophrenia.


The paper that I read in Lewis’ bookstore that afternoon was authored by Alexander Shulgin and his colleagues, Thornton Sargent and Claudio Naranjo. Although both Shulgin and Sargent were American, Naranjo was from Chile and the experiments had been carried out at the University of Chile in Santiago. What Shulgin and his colleagues had done was this: in order to begin elucidating how mescaline produced its effects, they had synthesized 44 different versions of the mescaline molecule, each one slightly different from the parent drug, and asked how this had changed its hallucinogenic activity. To answer this question they had done what I considered to be a very brave thing, and something that was completely in keeping with the zeitgeist of the time. They had tested each new chemical compound on themselves and, it was rumored, a number of students. Shulgin realized that the type of mental activity that was produced by hallucinogenic drugs like mescaline and LSD was something that was uniquely human. There are presumably effects that occur in animals as well, but they are not in a position to tell us about them. Even humans often struggle to describe the ineffable effects of psychedelics. It should be recalled that when Albert Hofmann discovered LSD in 1938, he tested it on mice and saw no obvious effect. It was only when he accidently ingested some of the drug himself several years later that he discovered its amazing properties. There is no adequate way of assessing the effects of psychedelic drugs except by testing them on humans. Even today, the behavioral assays that many scientists use involving mice to examine the effects of psychedelic drugs are mostly useless and don’t even represent eidolons of the actual experience.
Shulgin, of course, had realized this fact and so had traveled to Chile where the constraints on human drug testing were a little more “relaxed.” Shulgin subsequently became the high priest of psychedelic drug research in the 1960s and 1970s. He continued to prepare hundreds of mescaline derivatives at his home laboratory in California and tested them out on himself and his friends, eventually publishing all of his findings in his book “PIHKAL: A Chemical Love Story”—PIHKAL standing for “Phenethylamines I Have Known And Loved.” The book is considered to be one of the bibles of the psychedelic drug literature and describes the chemical synthesis of each drug along with notes as to the effects they produced on Shulgin and his friends. Shulgin discovered many important things about mescaline and also about drugs that were closely related to it from the chemical structural point of view, but which turned out to have different interesting properties. This included the molecule methylenedioxymethamphetamine (MDMA or Ecstasy), a psychotropic drug with a similar structure to mescaline but an entirely different mechanism of action, which produces a different spectrum of psychotropic effects. Shulgin continued along these lines until his death, but others have continued to make new versions of mescaline up until the present day seeking, perhaps, the ultimate psychedelic molecule, one so powerful that it could deconstruct all forms of normal thinking permanently after just one dose. Indeed, in a paper published just last month in the journal Cell (2), a group of scientists has used one of the latest versions of mescaline to probe the ultimate truths of its mechanism of action.
Like many drugs throughout history that have come to us from nature, the roots of mescaline use are extremely ancient; remains of mescaline-containing plants have been found in the Americas going back around 6,000 years. This was prior to the development of writing in Mesopotamia around 3–4,000 years ago, and so we don’t know what the drug was being used for at the time. However, we have every reason to believe, based on its subsequent history, that it was being used for shamanic purposes during religious ceremonies. Mescaline is obtained from certain types of cacti found in central and south America, particularly Lophophora williamsii (peyote cactus) and Echinopsis pachanoi (San Pedro cactus). The traditional method of consuming mescaline is to chew the dried tops of peyote cacti known as peyote buttons, although it is also used by making infusions and decoctions of various types.

The use of mescaline was unknown to Europeans until the Spanish conquistadors encountered it in the 15th century when they conquered the Aztec empire in Mexico. Indeed, the Spanish discovered that the indigenous American peoples used a wide variety of psychotropic agents in addition to mescaline. These included hallucinogenic mushrooms (psilocybin), morning glory seeds (lysergic acid amide), ayahuasca (N,N-dimethyltryptamine), tobacco (nicotine) and jimson weed (atropine/scopolamine). Their use was studied by the Spanish monk Bernardino de Sahagun. He learned the local language (Nahuatl) and taught his Aztec students Spanish and Latin and so could communicate with them very accurately and was able to compose a detailed account of their habits and beliefs, including the way they used hallucinogenic drugs in their religious ceremonies. De Sahagun used his findings to compose the Florentine Codex, one of the greatest works in the history of anthropology. He describes the use of mescaline as follows:
“There is another herb like mountain prickly pear,
named peiotl, which is white and can be found in the north.
Those who eat or drink of it see terrifying or absurd visions;
this inebriation lasts two or three days and then subsides.
It is a delicacy often enjoyed by the Chichimeca, for it is
sustaining and spurs them to fight with no thought of fear,
thirst, or hunger, and they say that it protects them from all
danger.”
De Sahagun aside, the conquistadors went out of their way to suppress the religious practices of the American peoples they encountered in a wholesale effort to convert them to Catholicism. As can be imagined, there was a great deal of reluctance to doing this and the use of hallucinogenic drugs was forced underground. Interestingly, Spanish proselytizing eventually resulted in the development of syncretic religions that were ostensibly Christian but maintained a good deal of their original American Indian character and incorporated the use of drugs like mescaline and psilocybin in the celebration of Christian ceremonies. However, very little was heard about these practices for several hundred years. During this time, the use of mescaline by indigenous peoples started to spread northwards and, by the 19th century, it was being used by “Indians” in the Texas/New Mexico/Arizona region. A few reports of its use began to circulate in the American press including, for example, a report that during the United States Civil War, Texas Rangers soaked peyote in water and drank the resulting hallucinogenic liquid.
In 1888 the pioneering psychopharmacologist, Louis Lewin, author of the book Phantastica, visited the United States and received a sample of a peyote cactus which he took home to Germany. He gave it to the Berlin Botanical Museum, who named it Anhalonium lewinii (although as we have seen the modern name is Lophophora williamsii). Lewin obtained a crude extract of the alkaloids contained in the cactus. Naturally, cacti like peyote contain thousands of chemicals including a large number of substances that are related to mescaline biosynthetically but are not psychoactive. Lewin probably succeeded in isolating some of these but not mescaline itself. However, in 1896, mescaline was isolated from peyote by Arthur Heffter, who also reported its psychedelic properties based on some self-experimentation. These reports stimulated a good deal of interest in mescaline and several people experimented with its psychotropic effects resulting in the publication of books such as Der Meskalinrausch—“The Mescaline Rush”—by Kurt Beringer. The pioneering sex psychologist Havelock Ellis extracted several peyote buttons, drank the resulting decoction and wrote a poetic account of his experience describing it as a “saturnalia of the specific senses, and chiefly an orgy of vision.” Mescaline was eventually synthesized by the Austrian pharmacologist Ernst Späth, in 1919, and from that point in time the pure substance was available for experimentation.
Widespread modern interest in the use of psychedelic drugs had to wait until after the Second World War. In particular, it was the publication of The Doors of Perception and the essay Heaven and Hell by Aldous Huxley in 1954/6 that introduced the properties of mescaline to the public at large. Indeed, the modern recognition of the properties of psychedelic drugs was well underway in the 1950s. In addition to Huxley’s books, the American banker and ethnobotanist Gordon Wasson journeyed to Mexico and “discovered” psilocybin use by Mexican Indians during religious ceremonies in out-of-the-way villages where they had secretly been using it since the time of the conquistadors. In 1957, he wrote a widely read essay in Life magazine entitled Seeking the Magic Mushroom. One of the people who read this essay was Timothy Leary, then a young faculty member in the psychology department at Harvard, who decided to go down to Mexico and try psilocybin out for himself. Leary was very impressed and when he returned to Harvard switched his research program to investigating the effects of hallucinogenic drugs. Topping all of this off, of course, was the discovery of the hallucinogenic effects of LSD by Albert Hofmann working at the Sandoz drug company in Basel in 1943.
The hallucinogenic drug revolution was now well on its way. One of major participants in this revolution was actually the CIA, who tested different psychotropic drugs, including mescaline, in their MK-ULTRA “brainwashing” program. They had received information that the Nazis had been testing the drug on concentration camp inmates during the war and even imported the Nazi scientist Kurt Plotner, who had performed the work, to help them out. By the early 1960s, however, it was clear that LSD was by far the most powerful hallucinogen available and it became the drug of choice by members of the counterculture movement of the 1960s. Of course, many people had read about mescaline, particularly from Huxley’s book, but nobody ever used it. When I was in the USA in 1969, I never encountered a single individual who had actually tried mescaline.
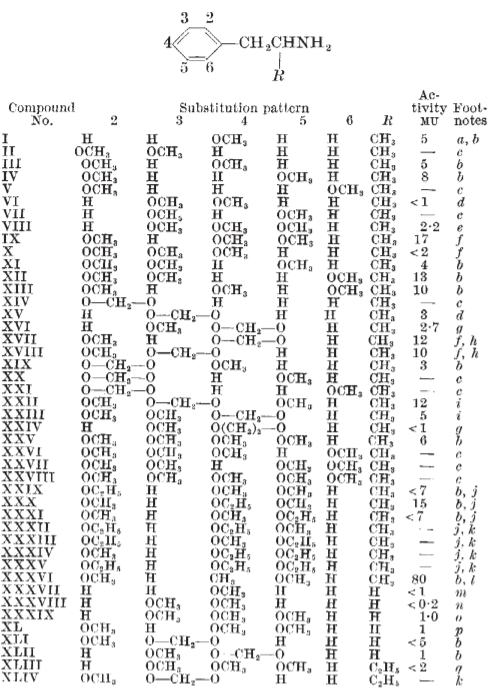
Mescaline is not that difficult to make. It is actually much easier to synthesize than LSD. The problem with mescaline is that it isn’t actually a very good psychedelic drug. If you want to trip you might take around 100 μg of LSD, but you would need more than 1,000 times that amount—over 100 mg—of mescaline to get a similar effect. So, when Shulgin performed the study he published in 1969, he quickly realized that it was quite simple to make improvements to the biological activity of the drug. You can already see some of these innovations in his 1969 paper. What happens if instead of mescaline, which is 3,4,5-phenethylamine, you make 3,4,5-amphetamine which is a very closely related structure in which the propylamine side chain has a methyl group added to it? This drug was twice as good as mescaline. Then how about the 3,4,5-methoxy groups—how necessary are they? The 2,4,5-methoxy analogue of amphetamine was even more effective, about 17 times as good as mescaline. Now what happens if you keep the 2,5-methoxy groups and put a methyl group rather than a methoxy in position 4? The resulting molecule named DOM turned out to be a whopping 80 times better than mescaline! If a bromine (Br, DOB) or iodine (I, DOI) atom was placed in the 4 position instead of the methyl group, the resulting compounds were even more potent. This was also true if one went back to the original mescaline-like phenylethylamine structure to yield substances like 2C-B. But that wasn’t the end of the story. What would happen if you traveled down to the other end of the molecule and started playing around by substituting the nitrogen atom? Addition of a benzyl group to 2C-B (25B-NB) produced a further increase in activity, and adding an ortho-methoxy to the benzyl group increased the activity even further. The resulting compound 25B-NBOMe is about 1,000 times more potent than mescaline and is even more potent than LSD, making it one of the most potent psychotropic substances ever made. In addition to their potency, some of these substances such as (S,S)-DMBMPP and 25CN-NBOH are highly selective at interacting with the 5-HT2A receptor, the most important mediator of the hallucinogenic effects of these drugs in the brain.

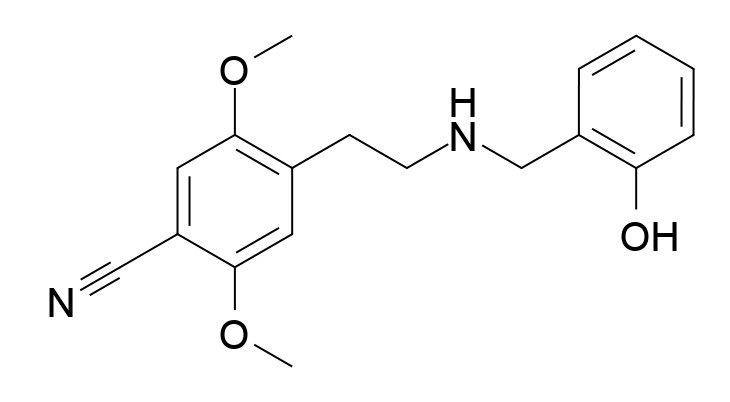
One has to wonder about such super-potent psychotropic drugs. Medicinal chemists are so sophisticated these days that they can usually achieve these kinds of results with any class of drugs that we originally discovered from nature. In the field of opioid drug research, for example, substances like some of the derivatives of fentanyl are thousands of times more potent than morphine and are extremely deadly as street drugs. Synthetic analogues of the archetypal cannabinoid drug tetrahydrocannabinol (THC) are also much more potent and have resulted in deadly effects as street drugs. In the last few years, hallucinogenic drugs like 25I-NBOMe and 25B-NBOMe have been reported as being used as street drugs under the name N-bombs. Serious overdose problems and some fatalities have been reported. Sophisticated medicinal chemistry together with the Internet is a very effective way of spreading new chemicals throughout society and there is no end of substances that can potentially be made, and perhaps no end to the potency that can be achieved. We can therefore see how mescaline has journeyed from being a religious sacrament thousands of years ago to becoming the blueprint for synthesizing some of the most potent hallucinogenic drugs ever made.

Whatever the future holds in store for these substances in recreational drug circles, their extreme potency and selectivity make them ideal tools for basic scientists investigating the mechanism of action of psychedelic drugs. As we have discussed, psychedelic drugs like LSD and mescaline interact with 5-HT2A receptors in the brain. These receptors are mostly expressed in the cerebral cortex, which makes them well placed for regulating phenomena connected with the higher cognitive functions of the brain. But how exactly does a drug like LSD activate a 5-HT2A receptor? Answering this question is clearly of considerable importance if you want to really understand how the drug works. To do this, it is important to study the chemical structure of the 5-HT2A receptor in its active conformation, that is when it is being activated by an agonist drug such as LSD. In their recent paper in the journal Cell, Kim et al. (2) achieved this aim. The authors decided to use two methods for conducting their studies, X-ray crystallography and a technique that is being used increasingly for structural studies of this type: “single particle cryo-electron microscopy (cryo-EM).” The results of these studies produced molecular structures at “near atomic resolution” and represent the most sophisticated structural picture of hallucinogenic drugs activating 5-HT2A receptors available to date. Not only are the results very interesting if you are a molecular pharmacologist, but they can also be appreciated by professionals and amateurs alike because of their aesthetic appeal, the figures and structures being presented in distinctly trippy hues of pink and purple. It should be noted that the drug chosen to activate the 5-HT2A receptor for the cryo-EM study was the highly selective and potent mescaline derivative 25CN-NBOH. The story of mescaline has followed an arc of discovery lasting many thousands of years. The drug has helped human beings discover themselves and scientists to discover the secrets of the brain. And so to the future when mescaline will surely continue to help us unlock the doors of perception.
References
1) Structure–activity relationships of one-ring psychotomimeticsShulgin AT, Sargent T, Naranjo C. Nature. 1969 Feb 8;221(5180):537-41. doi:10.1038/221537a0. PMID: 5789297
2) Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin ReceptorKim K, Che T, Panova O, DiBerto JF, Lyu J, Krumm BE, Wacker D, Robertson MJ, Seven AB, Nichols DE, Shoichet BK, Skiniotis G, Roth BL. Cell. 2020 Sep 17;182(6):1574-1588.e19. doi: 10.1016/j.cell.2020.08.024. PMID: 32946782
3) DARK Classics in Chemical Neuroscience: NBOMesPoulie CBM, Jensen AA, Halberstadt AL, Kristensen JL. ACS Chem Neurosci. 2019 Nov 12. doi: 10.1021/acschemneuro.9b00528. Online ahead of print. PMID: 31657895
